Gas chromatographs – The finest detection noses in the OELCHECK laboratory
Year of publication: 2020
Is a used engine oil contaminated with fuel? Has an engine oil been too heavily contaminated with biodiesel or vegetable oil? Has the wrong fuel been refuelled? Is there coolant in the oil sample? Does the DGA (dissolved gas analysis) of a transformer oil show a gas composition that indicates a likely fault in the transformer in the near future?
OELCHECK tribologists are confronted with such questions on a daily basis. Gas chromatography (GC) provides exact information to answer these questions as it can be used to analyse substance mixtures both quantitatively and qualitatively.
The prerequisite for an analysis is that the mostly liquid samples become gaseous without decomposition when heated or are already gaseous.
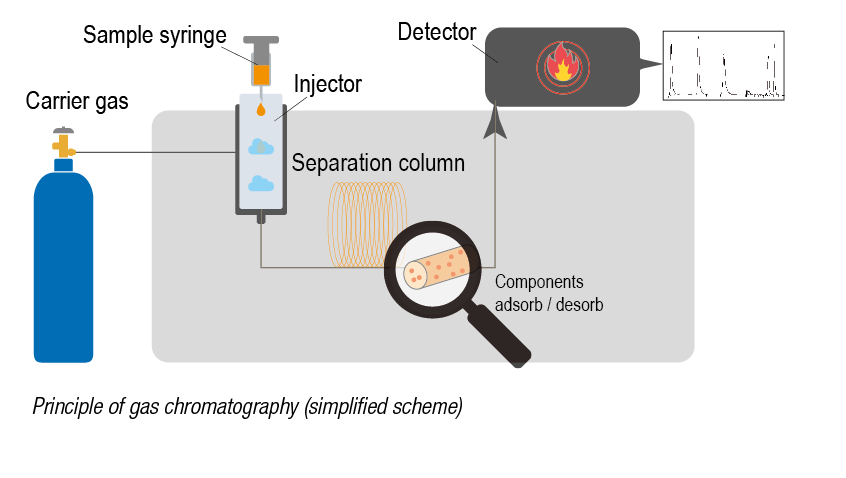
Four gas chromatographs are installed in the OELCHECK laboratory for analysis of up to 2,000 samples that are tested daily. Although set up for different purposes, they work according to a uniform principle. The sample to be examined is fed to the gas chromatograph via an injector. It then vaporises the sample. The now gaseous components are injected into a capillary column mounted in a thermally controllable oven. Gases pass through a long, thin glass tube with an inner diameter of less than 1 mm but with a length of up to 30 m. This is coated internally with a thin film, the stationary phase. A carrier gas such as hydrogen or argon, the mobile phase, continuously flows through this separation column. The gaseous components of the sample supplied from the injector remain at the stationary phase of the column for different lengths of time depending on their structure and the temperature in the oven chamber. As the individual components leave the column, a detector identifies them. They are recorded in a chromatogram and can be assigned to specific initial materials according to their boiling temperature. The later a component is detected at the column outlet, the higher its boiling point. The area under a peak in the chromatogram is proportional to the amount of the component in the mixture.