Flash point - Comparison of different analysis methods
The flashpoint is the key criterion for the flammability of combustible liquids. Combustible liquids includes fuels such as kerosene, conventional diesel fuel and biodiesel, whose classification into hazard classes is based on their flashpoint and more. However, the flashpoint also provides valuable assistance when inspecting engine oils and heat transfer media. If significantly lower, it may indicate that engine oil has mixed with a fuel or, in the case of heat transfer media, the formation of volatile cracked products and the associated increased risk of fire.
The flashpoint is defined as the lowest temperature at which a vapour phase formed over a liquid can be ignited with a test flame or electric igniter. Yet the sample itself doesn't burn – only the vapour phase does, and the flame then goes out again immediately.

Determination with small, closed crucibles
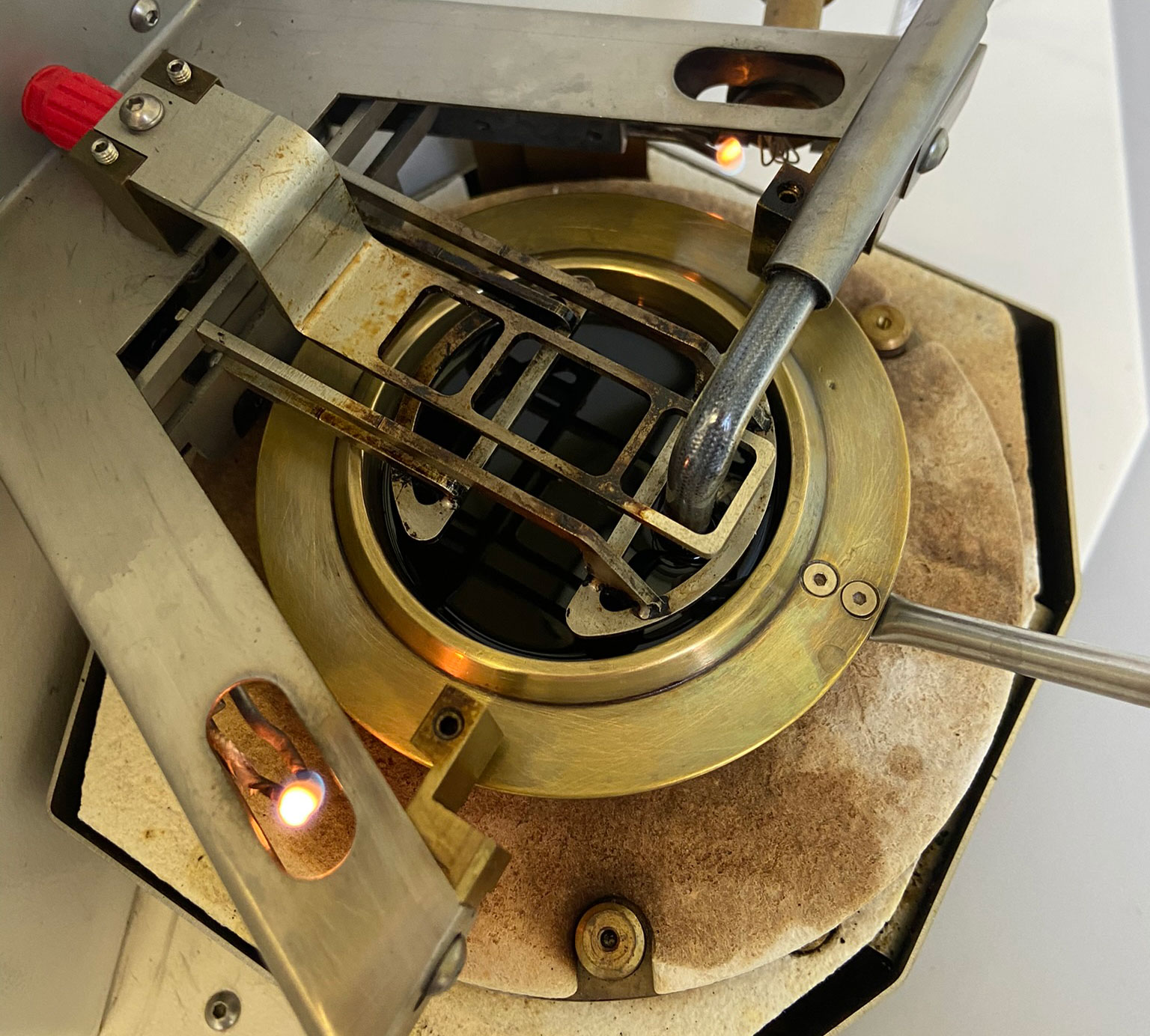
Determining the flashpoint is included in some OELCHECK all-inclusive analysis kits as standard. Setaflash flashpoint test devices with a small, closed crucible for a flashpoint in the range of 30-300°C are installed in our lab for this purpose.
We therefore determine the flashpoint pursuant to DIN EN ISO 3679, ASTM D3828 or ASTM D7236 in several incremental steps, which are then repeated to make sure we find the exact flashpoint. First, a sample quantity of 2ml (below 100°C) or 4ml (above 100°C) is placed into the crucible and tempered to the expected flashpoint temperature for 60 or 120 seconds. The tester's ignition device is then triggered. If a vapour phase has not formed at the temperature and cannot be ignited, the crucible is filled with a new sample quantity and tempered at a new test temperature. The next ignition attempt then follows, with this process repeated using clean sample quantities and different temperatures until the flashpoint is established.
Although the procedure is rather complex, determining the flashpoint using a small, closed crucible has several advantages:
- Volatile components in the sample do not go unnoticed. Our employees and the environment are protected from any harmful emissions.
- Only a small sample amount (30ml) is required.
- Each sample specimen is only tested once.
- The examination process is partially automated and therefore streamlined.

Alternative methods as additional tests
In addition to the small, closed crucible method, we determine the flashpoint using the Cleveland open crucible method (DIN EN ISO 2592, ASTM D92 / application of 79-400°C) and the Pensky-Martens closed crucible method (DIN EN ISO 2719, ASTM D93 / application of 40-370°C).
These are offered as additional tests to the all-inclusive analysis kits. However, the following must be considered:
- In the Cleveland method, the crucible is open. And according to the Pensky-Martens method, the larger, closed crucible must also be open for testing, too. In this process, parts of volatile components can escape without a flashpoint being detected.
- This means a minor ingress of fuel into an engine oil or an incipient accumulation of cracked products in heat transfer oils can quickly be overlooked.
- A sample volume of 200ml is therefore required during these test procedures, while a sample volume of 30ml is enough to determine the flashpoint with a small, closed crucible.